Iodine »
PDB 4k1c-4mw7 »
4lmn »
Iodine in PDB 4lmn: Crystal Structure of MEK1 Kinase Bound to GDC0973
Enzymatic activity of Crystal Structure of MEK1 Kinase Bound to GDC0973
All present enzymatic activity of Crystal Structure of MEK1 Kinase Bound to GDC0973:
2.7.12.2;
2.7.12.2;
Protein crystallography data
The structure of Crystal Structure of MEK1 Kinase Bound to GDC0973, PDB code: 4lmn
was solved by
M.H.Ultsch,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.69 / 2.80 |
| Space group | P 62 |
| Cell size a, b, c (Å), α, β, γ (°) | 81.610, 81.610, 129.809, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.1 / 23.3 |
Other elements in 4lmn:
The structure of Crystal Structure of MEK1 Kinase Bound to GDC0973 also contains other interesting chemical elements:
| Fluorine | (F) | 3 atoms |
| Magnesium | (Mg) | 1 atom |
Iodine Binding Sites:
The binding sites of Iodine atom in the Crystal Structure of MEK1 Kinase Bound to GDC0973
(pdb code 4lmn). This binding sites where shown within
5.0 Angstroms radius around Iodine atom.
In total only one binding site of Iodine was determined in the Crystal Structure of MEK1 Kinase Bound to GDC0973, PDB code: 4lmn:
In total only one binding site of Iodine was determined in the Crystal Structure of MEK1 Kinase Bound to GDC0973, PDB code: 4lmn:
Iodine binding site 1 out of 1 in 4lmn
Go back to
Iodine binding site 1 out
of 1 in the Crystal Structure of MEK1 Kinase Bound to GDC0973
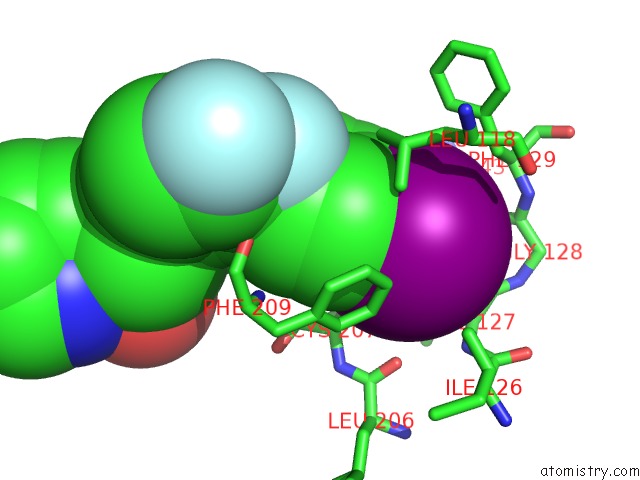
Mono view
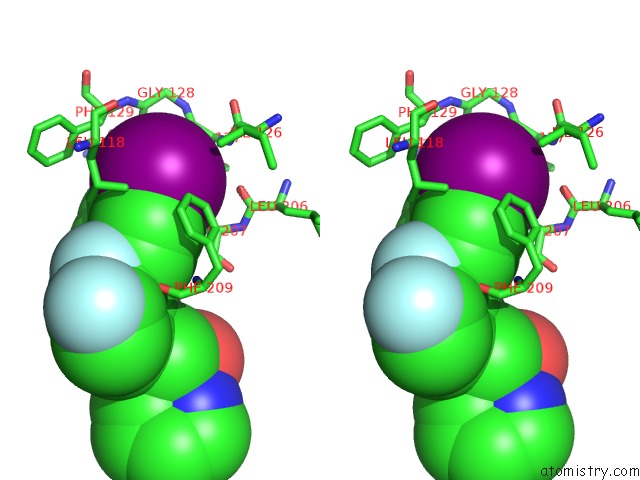
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iodine with other atoms in the I binding
site number 1 of Crystal Structure of MEK1 Kinase Bound to GDC0973 within 5.0Å range:
|
Reference:
G.Hatzivassiliou,
J.R.Haling,
H.Chen,
K.Song,
S.Price,
R.Heald,
J.F.Hewitt,
M.Zak,
A.Peck,
C.Orr,
M.Merchant,
K.P.Hoeflich,
J.Chan,
S.M.Luoh,
D.J.Anderson,
M.J.Ludlam,
C.Wiesmann,
M.Ultsch,
L.S.Friedman,
S.Malek,
M.Belvin.
Mechanism of Mek Inhibition Determines Efficacy in Mutant Kras- Versus Braf-Driven Cancers. Nature V. 501 232 2013.
ISSN: ISSN 0028-0836
PubMed: 23934108
DOI: 10.1038/NATURE12441
Page generated: Fri Aug 8 17:46:12 2025
ISSN: ISSN 0028-0836
PubMed: 23934108
DOI: 10.1038/NATURE12441
Last articles
Mg in 1W0JMg in 1W0H
Mg in 1VZM
Mg in 1VZK
Mg in 1VZ0
Mg in 1VTY
Mg in 1VTN
Mg in 1VST
Mg in 1VSD
Mg in 1VS0