Iodine »
PDB 1lkr-1pvh »
1nlu »
Iodine in PDB 1nlu: Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin
Enzymatic activity of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin
All present enzymatic activity of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin:
3.4.21.100;
3.4.21.100;
Protein crystallography data
The structure of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin, PDB code: 1nlu
was solved by
A.Wlodawer,
M.Li,
A.Gustchina,
Z.Dauter,
K.Uchida,
H.Oyama,
N.E.Glodfarb,
B.M.Dunn,
K.Oda,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.30 |
| Space group | P 62 |
| Cell size a, b, c (Å), α, β, γ (°) | 97.840, 97.840, 82.680, 90.00, 90.00, 120.00 |
| R / Rfree (%) | n/a / 19.5 |
Other elements in 1nlu:
The structure of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin also contains other interesting chemical elements:
| Calcium | (Ca) | 1 atom |
Iodine Binding Sites:
The binding sites of Iodine atom in the Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin
(pdb code 1nlu). This binding sites where shown within
5.0 Angstroms radius around Iodine atom.
In total 2 binding sites of Iodine where determined in the Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin, PDB code: 1nlu:
Jump to Iodine binding site number: 1; 2;
In total 2 binding sites of Iodine where determined in the Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin, PDB code: 1nlu:
Jump to Iodine binding site number: 1; 2;
Iodine binding site 1 out of 2 in 1nlu
Go back to
Iodine binding site 1 out
of 2 in the Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin
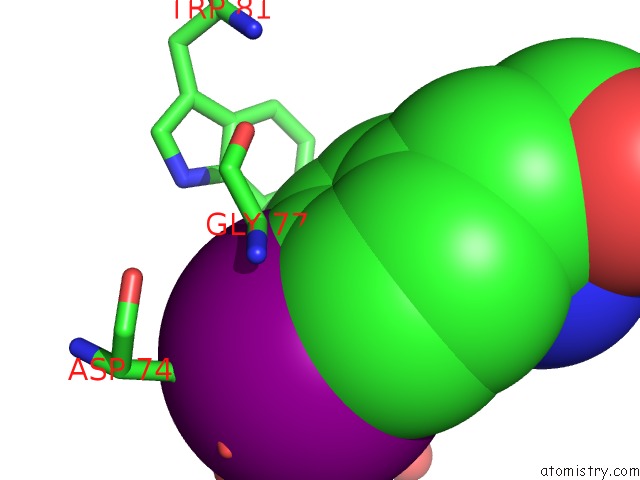
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iodine with other atoms in the I binding
site number 1 of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin within 5.0Å range:
|
Iodine binding site 2 out of 2 in 1nlu
Go back to
Iodine binding site 2 out
of 2 in the Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iodine with other atoms in the I binding
site number 2 of Pseudomonas Sedolisin (Serine-Carboxyl Proteinase) Complexed with Two Molecules of Pseudo-Iodotyrostatin within 5.0Å range:
|
Reference:
A.Wlodawer,
M.Li,
A.Gustchina,
H.Oyama,
K.Oda,
B.B.Beyer,
J.Clemente,
B.M.Dunn.
Two Inhibitor Molecules Bound in the Active Site of Pseudomonas Sedolisin: A Model For the Bi-Product Complex Following Cleavage of A Peptide Substrate. Biochem.Biophys.Res.Commun. V. 314 638 2004.
ISSN: ISSN 0006-291X
PubMed: 14733955
DOI: 10.1016/J.BBRC.2003.12.130
Page generated: Fri Aug 8 12:05:20 2025
ISSN: ISSN 0006-291X
PubMed: 14733955
DOI: 10.1016/J.BBRC.2003.12.130
Last articles
I in 5ENMI in 5ENK
I in 5EEK
I in 5E9O
I in 5EMS
I in 5EIJ
I in 5EI1
I in 5DLV
I in 5EHB
I in 5E5Y